AlphaFold and the Future of Precision Drugs
For decades, biology has faced a fundamental challenge: understanding how the information encoded in our genes translates into the physical machinery of life….
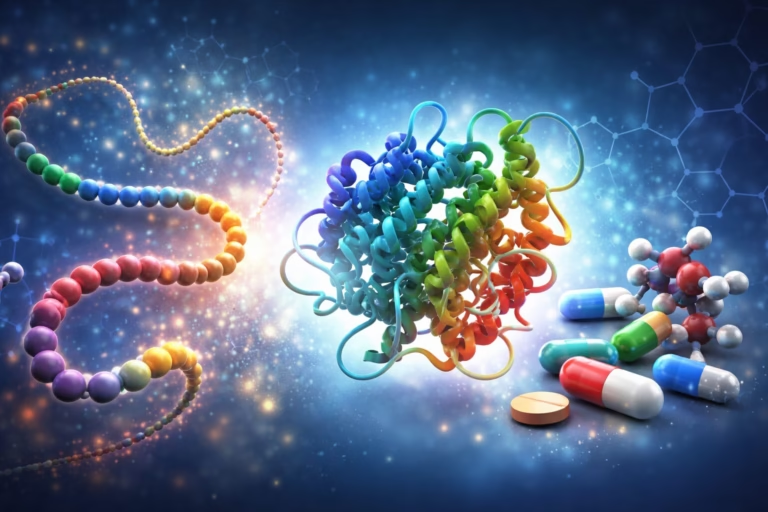
For decades, biology has faced a fundamental challenge: understanding how the information encoded in our genes translates into the physical machinery of life….

The UK has taken a major step forward in precision prescribing with the publication of its first national pharmacogenomic guideline, focused on the…

OneOme, a leading name in pharmacogenomics testing and personalized medicine, has announced that it will cease operations by November 14, 2025, citing ongoing…
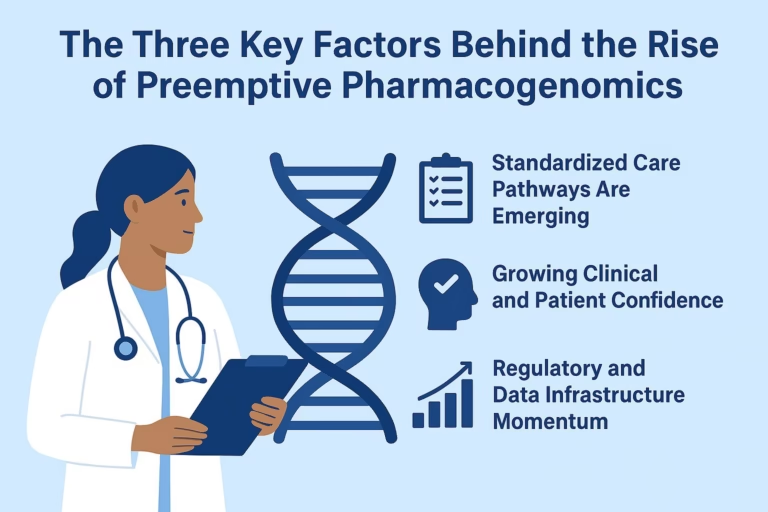
For years, pharmacogenomics (PGx) was seen as a future promise, a fascinating science that could someday make drug therapy safer and more effective….

In May 2024, the U.S. Food and Drug Administration (FDA) published a final rule that would bring laboratory-developed tests (LDTs) explicitly under the…

When you talk to Dr. Gualberto Ruaño, you don’t just hear about science — you hear about purpose. A physician, geneticist, and entrepreneur,…

Grand View Research has just released its latest report on the pharmacogenomics technology market, offering fresh insights into one of the fastest-growing areas…
Pharmacogenetic testing has emerged as one of the most exciting frontiers in personalized medicine. By analyzing a patient’s genetic makeup, these tests promise…
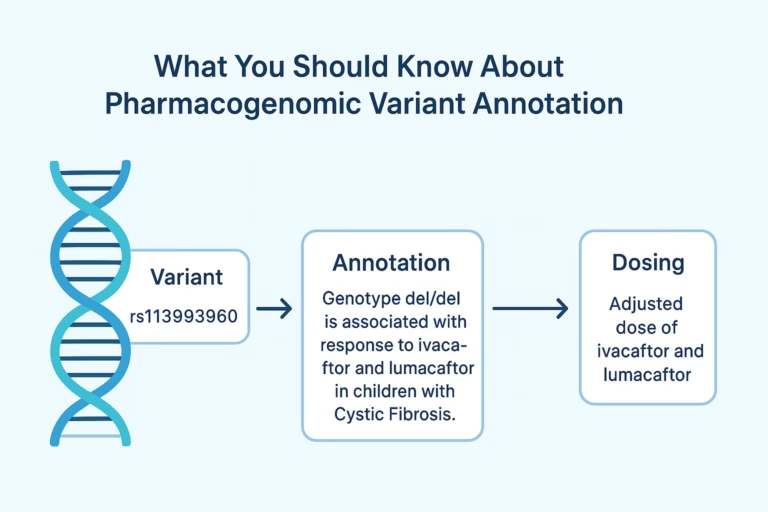
The promise of precision medicine lies in its ability to tailor treatments to the genetic makeup of each patient. Pharmacogenomics is central to…

Get the facts on Reimbursement for Pharmacogenomic Testing. Our case study offers a practical guide for providers and labs on navigating insurance and billing.
End of content
End of content
Join Our Insider’s Circle! Stay up to Date with the latest in PGx