The Biggest Pharmacogenomics Advances of 2025 — Year in Review
As 2025 comes to a close, pharmacogenomics has firmly stepped out of its former niche and into the spotlight as a research-driven catalyst…

As 2025 comes to a close, pharmacogenomics has firmly stepped out of its former niche and into the spotlight as a research-driven catalyst…

Pharmacogenomics—the study of how an individual’s genetic makeup influences their response to medications—has become one of the most important pillars of modern personalized…

In the last decade, pharmacogenomics (PGx) has moved from a niche research domain into a central pillar of precision medicine. Sequencing is now…

In May 2024, the U.S. Food and Drug Administration (FDA) published a final rule that would bring laboratory-developed tests (LDTs) explicitly under the…
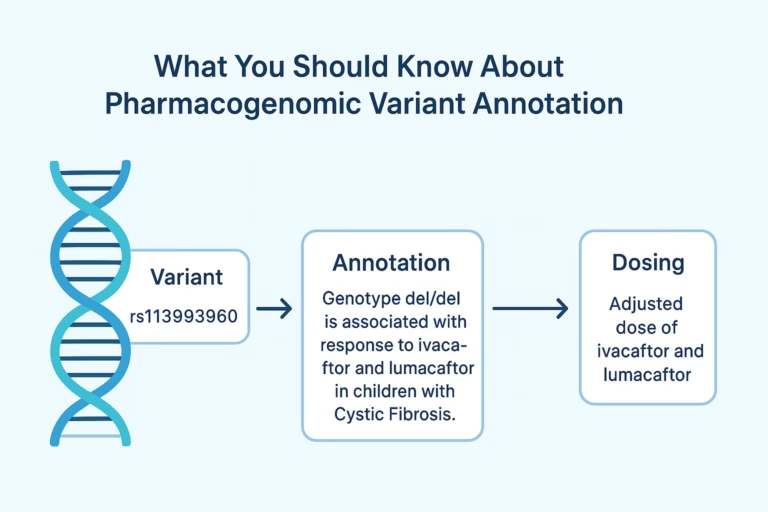
The promise of precision medicine lies in its ability to tailor treatments to the genetic makeup of each patient. Pharmacogenomics is central to…

This blog provides a comprehensive overview of the FDA’s current and draft guidance on pharmacogenomic data submission, highlighting key regulatory requirements, submission algorithms, voluntary submissions, and practical considerations for clinicians and industry professionals.

Learn about the barriers to Implementing PGx Testing

In this guide, we’ll explore what PGS are, how they are built, the value they bring to healthcare, and the challenges that come with them. We’ll then look at how they connect to pharmacogenomics—a field that uses genetics to tailor medication—and why learning both will be key for the future of medicine.

Dr. Patricia Cammazola doesn’t think so. “Physicians/prescribers are often not aware of the liability associated with not considering PGx FDA recommendations and CPIC…
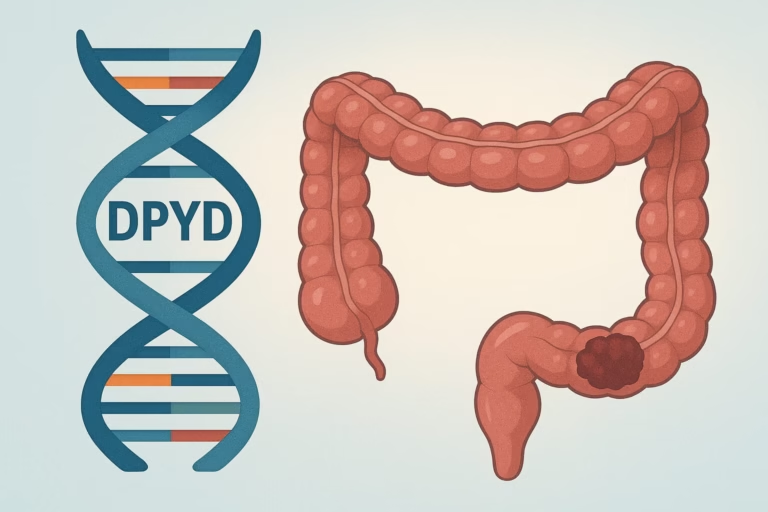
The key lies in the DPYD gene, which produces an enzyme called DPD that helps the body break down and clear fluoropyrimidines.
End of content
End of content
Join Our Insider’s Circle! Stay up to Date with the latest in PGx